find the number of electrons ga|Chemical Elements.com : Tuguegarao The electron configuration for Gallium, Ga is. 1s22s22p63s23p63d104s24p1. Explanation: Gallium, Ga has 31 protons and 31 electrons. The superscripts represent . Dark Green Lantern Male 21st Birthday Invitation. Invitation by Canva Creative Studio. 1 of 2. Invitación de cumpleaños juguetona, ilustración de mujer, purpura, negro y mostaza . To further personalize your free 21st .
PH0 · What is the electron configuration of the gallium ion?
PH1 · Protons, Neutrons, Electrons for Gallium(Ga, Ga3+)
PH2 · How to Find the Valence Electrons for Gallium (Ga)
PH3 · Gallium Valence Electrons
PH4 · Gallium
PH5 · Find the Number of Electrons Ga
PH6 · Chemical Elements.com
PH7 · 8.3: Electron Configurations
PH8 · 10.6: Valence Electrons
1K means in Hindi – 1M means in Hindi – तो दोस्तों जैसा कि अब Internet की दुनिया में हम सभी Social Media जैसे Facebook, YouTube और Instagram का इस्तेमाल बहुत अच्छे से करने लगे हैं। यहां तक कि कई लोग इन .
find the number of electrons ga*******There are two ways to find the number of valence electrons in Gallium (Ga). The first is to use the Periodic Table to figure out how many electrons Gallium has in its valence shell. To do.To find the number of electrons in gallium gallium, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. .
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom . The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom .Chemical Elements.com Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at .
The electron configuration for Gallium, Ga is. 1s22s22p63s23p63d104s24p1. Explanation: Gallium, Ga has 31 protons and 31 electrons. The superscripts represent . For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined). . Gallium Valence Electrons | Gallium Valency (Ga) with Dot Diagram. January 27, 2021 Leave a Comment. Learn everything about Gallium valence electrons .Number of Protons/Electrons: 31. Number of Neutrons: 39. Classification: Other Metals. Crystal Structure: Orthorhombic. Density @ 293 K: 5.907 g/cm 3. Color: White/Silver. . Introduction to Chemistry. 10: Electrons in Atoms. 10.6: Valence Electrons. Expand/collapse global location. 10.6: Valence Electrons. Page ID. ⚙️ Learning .
find the number of electrons ga Chemical Elements.com Using the average atomic mass, calculate the average number of neutrons by first rounding the atomic mass from 200.592 to 201. Now, subtract the number of protons, 80, from the atomic mass, 201-80, to find the average number of neutrons, 121. If the mass number of an isotope is known, the actual number of neutrons can be .
Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a mass of 1 atomic mass unit (amu) ( amu), which is about 1.67 ×10−27 1.67 × 10 − 27 kilograms. Together with neutrons, they make up virtually all of the mass of an atom.
First, to find the number of protons, we need to realize that the neutral atom had 53 electrons because it is the additional one electron that makes it a 1- anion. Now, because the atom has 53 electrons, it must also have 53 protons, and to find the number of neutrons we subtract this from the mass number. # n = A – # p = 127 – 53 = 74 .
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.Finding the Number of Neutrons. The number of neutrons in an atom can be calculated by subtracting the atomic number from the atomic mass. Both of these numbers can be found on the periodic table. The atomic number is listed above the symbol of the element whereas the mass number is placed below. Let’s keep using oxygen as our example.

There are two ways to find the number of valence electrons in Gallium (Ga). The first is to use the Periodic Table to figure out how many electrons Gallium h.
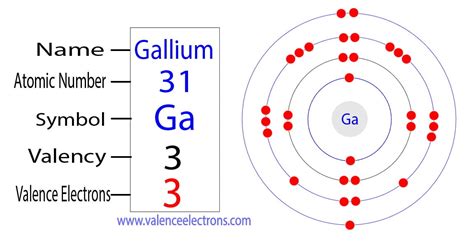
And you have one more electron to worry about. And so that electron would go into a 3S orbital. So the full electron configuration is 1S2, 2S2, 2P6, and 3S1. When I want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the . 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of gallium (Ga) is 31. Hence the gallium element has electrons arrangement 2, 8, 18, 3. This electron arrangement indicates that the outermost orbit of Gallium element (Ga) has 3 electrons.
Method 1: From the Periodic Table. To find out the valence electrons of Gallium, you have to see the position of gallium in the periodic table. More specifically, you have to see the group wise position of Gallium element in the periodic table. From the above image, you can see that the Gallium (Ga) is present in the group 13 of periodic table. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104.Gallium has the following electron configuration. Ga: [Ar] 4s 2 3d 10 4p 1. The 4s and 4p electrons can be lost in a chemical reaction, . Determine the number of valence electrons in neutral atoms of the following elements: (a) Si (b) Mn (c) Sb (d) Pb. Click here to check your answer to Practice Problem 1. The Covalent Bond. Atoms can combine .find the number of electrons ga The electron configuration for Gallium, Ga is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^1 Gallium, Ga has 31 protons and 31 electrons. The superscripts represent the electrons present in each region of the periodic table. The sum of these superscripts should equal the atomic number for a neutral atom. The last electron is in the 4th . Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
Sodium (Na) is the first element in the 3rd row (Period 3) in the periodic table. This means that the first shell and second shells of Na atom are filled to the maximum number of electrons. The first shell (1s) is filled with 2 electrons. The second shell (2s and 2p) has a total of 8 electrons. And, the third (last) shell has 1 electron.An Isoelectronic Series is a group of atoms/ions that have the same number of electrons. Examples. N 3-, O 2-, F-, Ne, Na +, Mg 2+, Al 3+ This series each have 10 electrons. P 3-, S 2-, Cl-, Ar, K +, Ca 2+, Sc 3+ This series each have 18 electrons. A typical question about isoelectronic series usually involve size comparisons. Since the .
Mass numbers of typical isotopes of Gallium are 69; 71. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the .Atomic Number: Ga: Element Symbol: Gallium: Element Name: 69.72: Average Atomic Mass: Step 2. To find the number of electrons in , first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since 's atomic number is , has electrons.
Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. Electrons are arranged around atoms in a special way. If you need to know how the electrons are arranged around an atom, take a look at the 'How do I read an electron configuration table?' page.
We'll walk you through what the best Workstation PC for DaVinci Resolve Studio looks like. Forum; PC Builds. . DaVinci Resolve is a powerful video editing package in a unique market position. . Of particular note is the emphasis they place on motherboard choices and PCIe speeds, as well as the RAM and VRAM .
find the number of electrons ga|Chemical Elements.com